|
Lipid Binding properties of AP180 and CALM
|

|
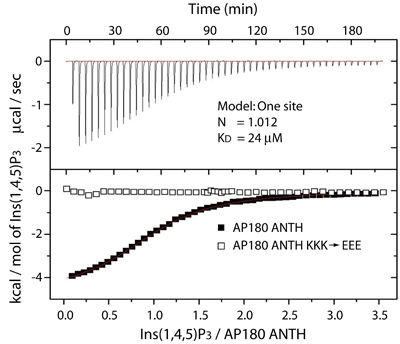
ANTH domain affinity forPtdIns(4,5)P2
Measurements by isothermal titration calorimetry show a 24uM affinity of the AP180 ANTH domain for Ins(1,4,5)P3...
...We measure the same affinity for binding to liposomes or to lipid tubules containing 10% PtdIns(4,5)P2. Thus only the head group of PtdIns(4,5)P2 is interacting, as in the crystal structure.
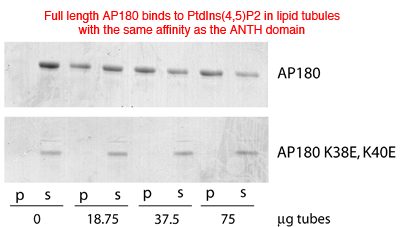
Full length AP180 also has the same affinity for liposomes and thus there are no additional lipid interactions. Mutations of lysines involved in PtdIns(4,5)P2 coordination abolish the interaction.
A 24uM affinity is weak, but clathrin (its interacting partner) is a triskelion and thus its interaction with membranes relies on the combined affinities of 3xAP180 molecules (see experiments on clathrin avidity below).
|
|
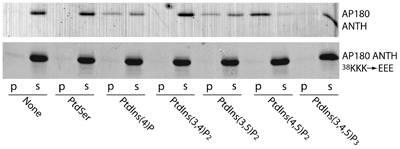  |
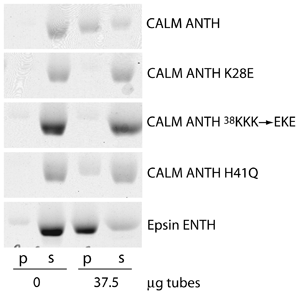
Lipid Specificity of ANTH domain
Binds best to PtdIns(4,5)P2, but also interacts with PtdIns4P and PtdIns(3,5)P2. It is surprising from a structural point of view why it does not interact with PtdIns(3,4,5)P3, but this result was confirmed by direct affinity measurements using the headgroup Ins(1,3,4,5)P4 which bound with a much lower affinity. Numbers in lower panel refer to labelled lanes in upper panel.
|
|
|
|
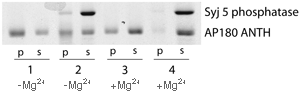
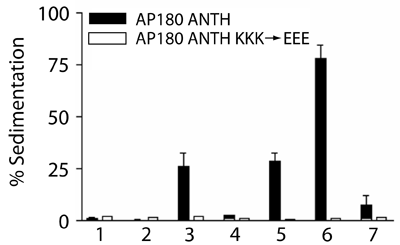
Inositol lipid 5' phosphatase uncoats the ANTH domain
Active synaptojanin (column 4) removes the ANTH domain from PtdIns(4,5)P2 containing liposomes, while inactive synaptojanin (absence of Mg2+, column 2) does not. There also be some interaction between synaptojanin and AP180 as some synaptojanin is found in the pellet in column 2. (1mM Mg is sufficient to activate synaptojanin 5'phosphatase activity).
|
|
|
|
CALM ANTH domain binds to PtdIns(4,5)P2
Clathrin assembly lymphoid myeloid leukemia protein (CALM) is a homologue of AP180 whose ANTH domain shares 81% sequence homology. Mutations of headgroup binding residues disrupt binding to lipid tubules containing 10% PtdIns(4,5)P2. The epsin ENTH domain also binds to PtdIns(4,5)P2.
|
|
|
|
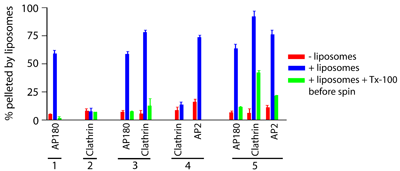
The influence of AP180 on AP2 and clathrin interactions with membranes
AP2 alone does not strongly promote clathrin association with membranes, while AP180 very strongly recruits clathrin to membranes. In this AP180 + clathrin experiment the ratio of clathrin:AP180 in the liposome pellet (+ tubes) is much greater than in the supernatant. This demonstrates the high avidity of clathrin for AP180 bound to membranes (see above), and is partly due to the effect of AP180 in promoting clathrin polymerisation. On further titration we showed that increasing the concentration of clathrin allowed us to saturate the membrane bound AP180 at a 1:1 stoichiometry (as assessed by Coomassie staining) and above this concentration the remaining clathrin was found in the supernatant.
|
|
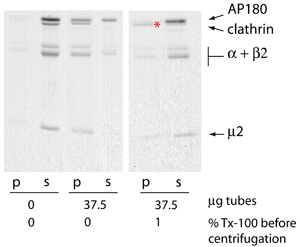 |
AP180 causes clathrin polymerisation on membranes
After dissolving the liposomes with Tx-100, polymerised clathrin remains assembled while AP180 and AP2 move back to the supernatant. We confirmed that clathrin was indeed polymerised in the presence of AP180 and membranes using electron microscopy.
|
|